NAC-GED-0507 is a Phase 3 ready topical treatment of Acne. NAC-GED-0507 is also formulated as a cream, has been tested in a Phase 1 clinical trial in patients affected by Mild-to-Moderate Psoriasis. Based on the positive results, a foam formulation (which has a higher potential market competitiveness) has been developed, and will be tested in upcoming clinical studies in patients affected by Psoriasis.

In silico screening
NAC-GED-0507 derives from a virtual library of molecules, rationally designed and screened in silico to obtain a new agent for the treatment of Immune-Inflammatory-Mediated Diseases, with anti-inflammatory activity and a very favourable safety profile.
Timeline
Target ID
Lead ID & Preclinical
Formulation Development
Clinical Phase l
Clinical Phase lI
Clinical Phase lII
Market
NAC-GED-0507

Lead Compound
NAC-GED-0507 was selected as Lead Compound for development in the Dermatological field, based on:
- its chemical structure, that makes it suitable for the development of robust semi-solid formulations for the topical treatment of dermatological disorders;
- its demonstrated in vitro nonclinical pharmacology activity on keratinocytes and sebocytes.
Topical Treatment of Acne
N-Acetyl-GED-0507-34-Levo (NAC-GED-0507) is a patent protected small molecule, that Nogra Pharma is developing for the topical treatment of Acne, formulated as hydrophilic, non-alcoholic gel.
The product is ready to enter Phase 3 (see list of CTs) as the development plans up to NDA /MAA have been agreed with FDA (EOP2 Meeting) and EMA.
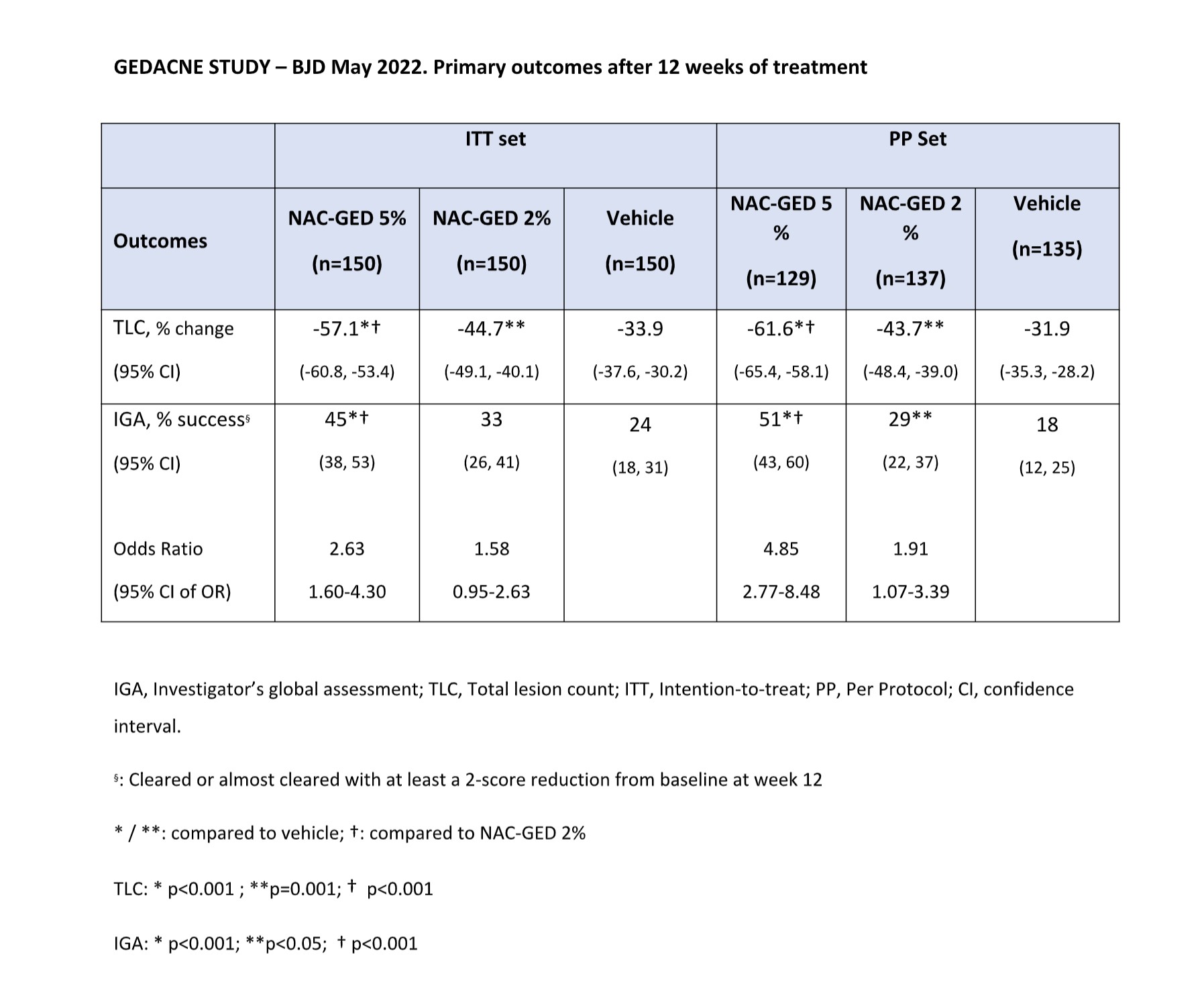
The Results of a large Phase 2b study (GEDACNE Study, 450 patients) have been published on British Journal of Dermatology in May 2022 (https://doi.org/10.1111/bjd.21663).
Topical application of NAC-GED significantly improves acne manifestations in patients with moderate-to-severe Acne and is safe and well-tolerated. (see Phase 2b Results).

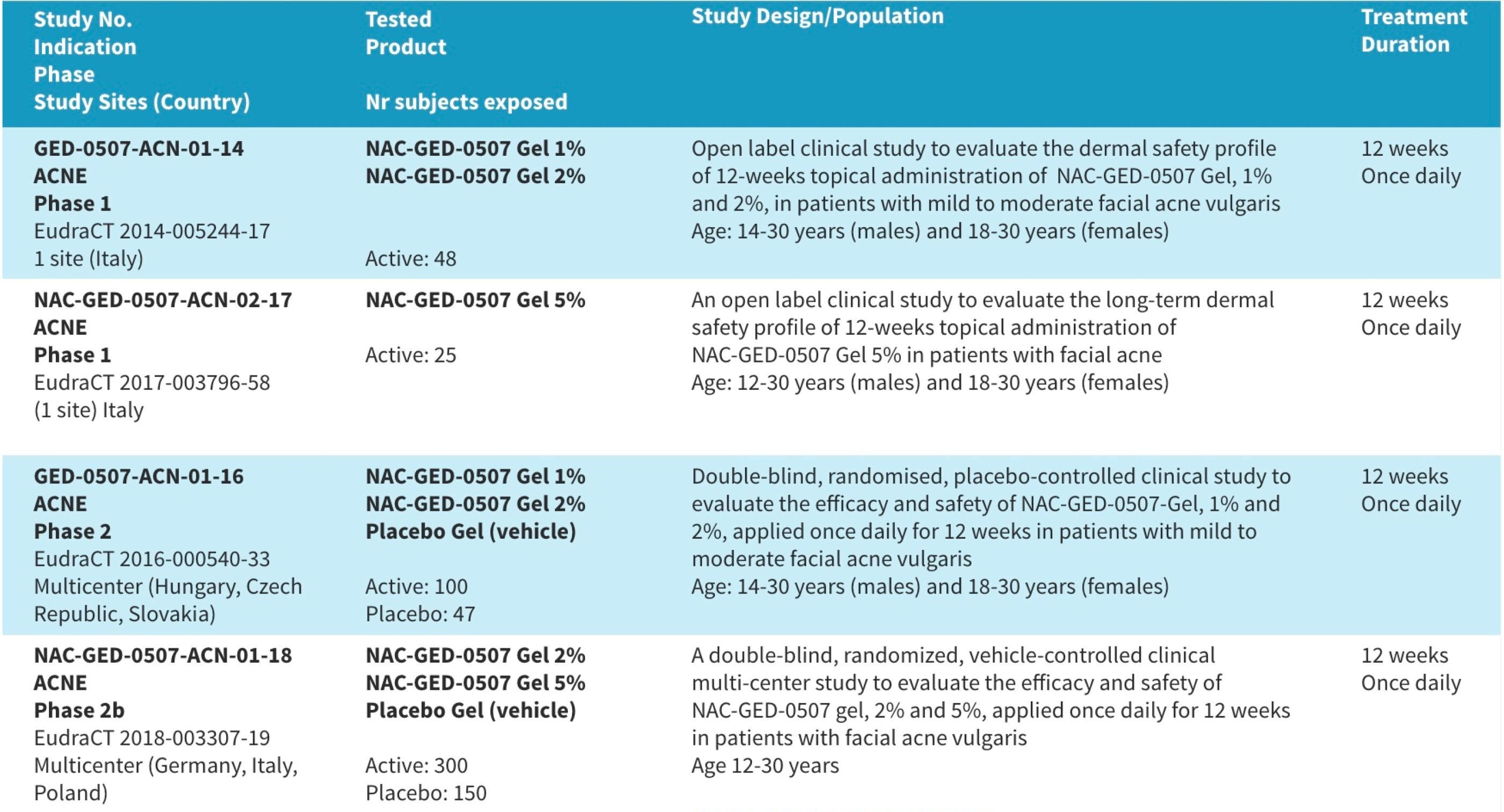
Performed Clinical Trials with NAC-GED-0507
Topical Treatment of Psoriasis
NAC-GED-0507, formulated as a cream, has been tested in a Phase 1 clinical trial in patients affected by mild-to-moderate Psoriasis.
Based on the positive results, a foam formulation (which has a higher potential market competitiveness) has been developed, and will be tested in upcoming clinical studies in patients affected by Psoriasis.