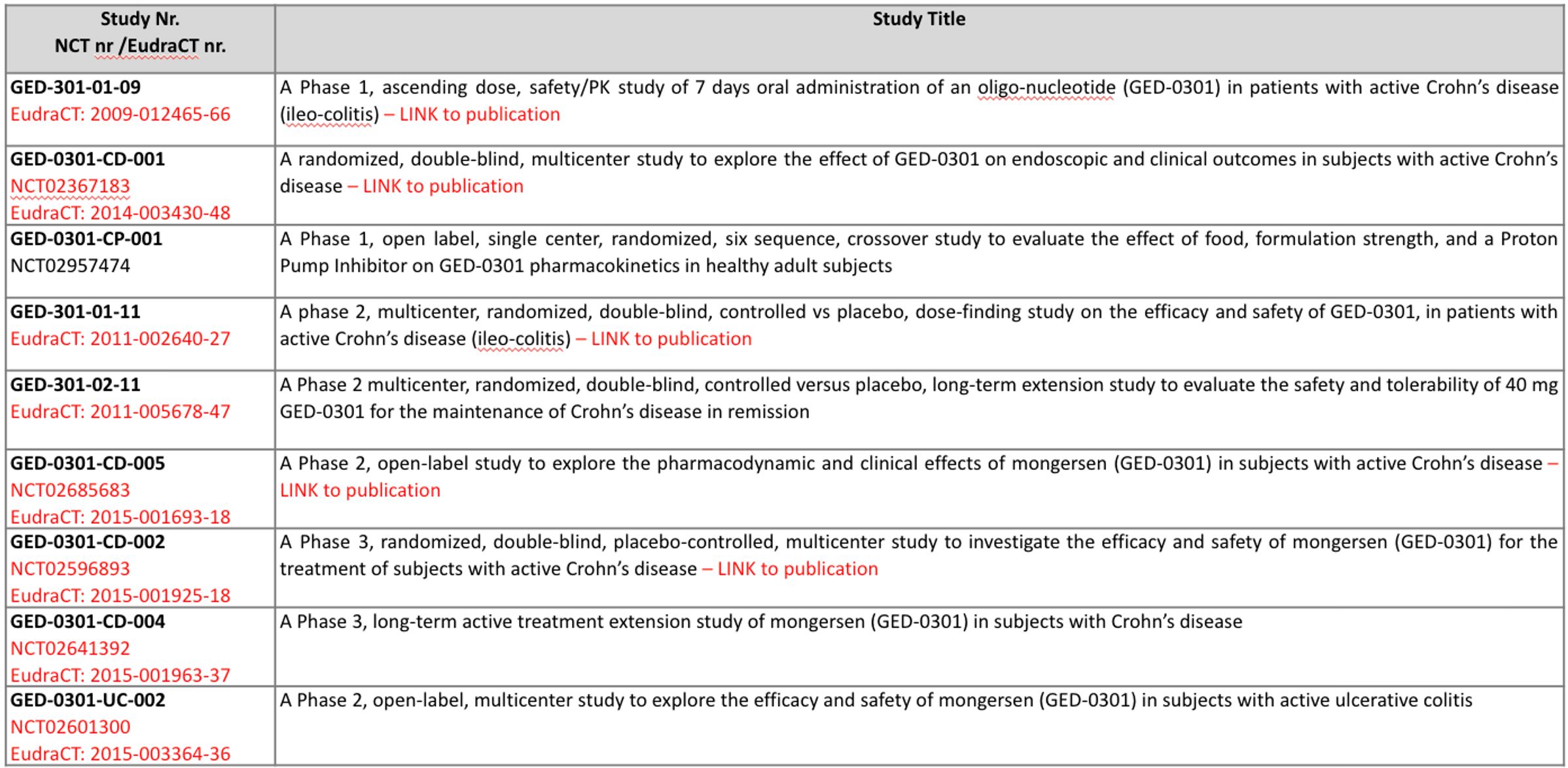
GED-0301 antisense oligonucleotide for the treatment of Inflammatory Bowel Diseases

High Level Overview
GED-0301 (Mongersen) is an antisense oligonucleotide (ASO), complementary to Smad7 mRNA, in clinical development for the treatment of Inflammatory Bowel Diseases (IBD).
For the oral treatment of Crohn’s Disease (CD), Mongersen is formulated as a gastro-resistant delayed-release tablet, designed to deliver the ASO in the terminal ileum and right colon, with negligible systemic absorption.
The Mongersen tablet is in clinical development and has been tested in more than 1000 patients affected by active CD, proving safe and well tolerated.
Development status
Target ID
Lead ID & Preclinical
Formulation Development
Clinical Phase l
Clinical Phase lI
Clinical Phase lII
Market
GED-0301
GED-0301 – History
- 2003-2014. Nogra Pharma developed the product from target identification up to a Phase 2 PoC double-blind placebo-controlled study in mild-to-moderate active Crohn’s Disease.
At the highest tested dose, in the PoC study, treatment resulted in a 65% clinical remission rate (P<0.001) and in a 72% clinical response rate (P<0.001). - (https://doi.org/10.1056/NEJMoa1407250).
- 2014. The Product is out-licensed to Celgene.
Clinical development continued, manufacturing scale-up for large clinical /commercial supply is performed and the nonclinical toxicology package for NDA is completed. The Product efficacy is confirmed in two additional clinical studies, including an endoscopy trial which also confirmed endoscopic efficacy of Mongersen. - (https://doi.org/10.1053/j.gastro.2017.08.035), (https://doi.org/10.1038/mt.2011.290)
- 2017. The development of Mongersen is halted based on a futility analysis performed during the Phase 3 study, which showed lack of efficacy.
- (htpps://doi.org/10.14309/ajg.0000000000000493).
- 2020. Nogra Pharma reacquired Mongersen.
- 2020-2022. Nogra Pharma performed a thorough investigation aimed at identifying the reasons for the failure of the Phase 3 clinical study despite the previous clinical trials documenting the efficacy of the drug, and determined that the Mongersen batches administered in clinical studies were not homogeneous and presented a different ability to down regulate SMAD7 in cultured cells.
- (https://doi.org//10.1089/nat.2021.0089), (https://doi.org/10.1007/s40291-022-00606-1), (https://doi.org/10.1007/s40259-021-00482-x), (https://doi.org/10.3390/pharmaceutics15010095).
- 2022. Upon identification of the source of the Phase 3 failure, Nogra Pharma has reactivated the development of Mongersen for the treatment of Inflammatory Bowel Diseases and further Clinical Study confirming original PoC Trial will be started in 2023

GED-0301 – Mongersen- Product Profile
Target: Smad7 mRNA
Drug Substance: antisense oligonucleotide complementary to Smad7 mRNA
Drug Product: GED-0301 gastro-resistant delayed release tablet for oral administration.
MoA: Mongersen ASO inhibits the expression of Smad7, a protein overexpressed in Inflammatory Bowel Diseases (Crohn’s Disease, Ulcerative Colitis), thereby restoring the physiological immuno-suppressive activity of TGF-β1 in the intestinal mucosa.
Indication: Crohn’s disease (CD)
Induction of Remission in CD
Maintenance of Remission in CD
Label Expansion: Paediatric CD, ulcerative colitis
Development Phase: Clinical; Mongersen tablet has been tested in more than 1000 patients affected by active CD. Further Clinical Study confirming original PoC Trial will be started in 2023.
Nonclinical toxicology: package for NDA/MAA completed
IP: Patent portfolio has been expanded to provide composition coverage beyond 2040

GED-0301 – Mongersen- SUMMARY & OUTLOOK
Drug Substance:
Following the characterization of the batches used in the clinical studies, the manufacturing optimization is ongoing.
(https://doi.org//10.1089/nat.2021.0089)
Drug Product:
The oral formulation, gastro-resistant delayed release tablets, 40 mg dose strength, already tested in CTs, is ready to be manufactured and tested in new upcoming clinical studies in patients affected by Crohn’s Disease.
GED-0301 has been fully characterized in a package of nonclinical safety studies. The package is complete to support NDA/MAA.
The clinical development plan foresees confirmatory studies in CD patients, to be activated in 2023.
Patent portfolio has been expanded to provide composition coverage beyond 2040.

The target Smad7
Inflammatory bowel diseases (IBD), are chronic, relapsing-remitting, immune-mediated disorders. The two main forms of IBD are Crohn’s Disease (CD) and Ulcerative Colitis (UC). The IBD-associated inflammatory process is sustained by defects of normal counter-regulatory mechanisms.
Professor Monteleone of University of Tor Vergata in Rome (Principal investigator of GED-0301) discovered that in the inflamed intestinal mucosa of patients with CD and UC, there is a defective signaling of TGFβ1 (a cytokine which acts as a potent regulator of intestinal immune-homestasis), due to high levels of Smad7 (a protein which negatively regulates TGFβ1 signaling).
(https://doi.org/10.1172/JCI12821)
In the last two decades, many studies have shed light on the pathogenetic role of Smad7 in the gastrointestinal tract and the mechanisms by which Smad7 sustains gut inflammation, supporting targeting Smad7 as a therapeutic approach to help attenuate detrimental immune-inflammatory responses in IBD.
(https://doi.org/10.1016/j.crimmu.2023.100055)

Therapeutic Approach
Prof. Monteleone demonstrated that inhibition of Smad7 expression with a Smad7 ASO, restored TGF-β1 activity, thereby inhibiting proinflammatory cytokines production in isolated lamina propria mononuclear cells (LPMC) from patients with CD and UC
(https://doi.org/10.1172/JCI12821).
This prompted Nogra Pharma to develop a Smad7 ASO for the treatment of IBD.
Mongersen is a single stranded, phosphorothioate ASO complementary to a sequence in Smad7 mRNA 100% homologous in humans, mice, monkeys.

In vivo Nonclinical Pharmacology and Formulation Development
GED-0301 was tested in the TNBS- and DSS-induced colitis mouse models, two widely used models that show striking similarities with human CD and UC, respectively.
In both models, GED-0301 ASO down-regulates Smad7 and restores TGF-β1signaling, decreases the synthesis of inflammatory molecules, the extent of colitis and gut damage.
(https://doi.org/10.1053/j.gastro.2006.09.016)
In particular, in the TNBS-induced colitis model, upon a single dose administration by oral gavage GED-0301 reaches the gut, where it is taken up by epithelial and lamina propria cells in the proximal small intestine, terminal ileum, and colon, reduces the mucosal expression of Smad7 “enhances TGF-β1-associated Smad2/3 phosphorylation” attenuates colitis.
Therefore activity of GED-0301 can be obtained by oral-local delivery of the ASO in the intestine. This prompted Nogra to develop a proprietary oral formulation, designed to deliver GED-0301 in the terminal ileum and colon of patients affectd by CD, with negligible systemic absorption

Clinical Data
In Phase 1, 2 and 3 Clinical Trials, more than 1000 patients affected by Inflammatory Bowel Diseases (Crohn’s Disease, Ulcerative Colitis) have been treated with Mongersen gastro-resistant delayed release tablets. In addition, a study in healthy volunteers to investigate the effect of food on GED-0301 PK, was performed.
In Summary:
- Safety. Mongersen was safe and well tolerated in all studies at doses up to 160 mg/day, administered for up to 12 consecutive weeks (induction phase) and up to 52 consecutive weeks (maintenance phase).
- PK. Negligible systemic absorption was confirmed (in line with the developed formulation).
- Efficacy. Due to discontinuation of the Program in 2017, conclusions on the efficacy of GED-0301 in inducing remission/response in patients affected by active Crohn’s Disease (first indication) can only be drawn from 5 clinical studies. Data of these studies were published. While the Phase 1 and 2 clinical studies documented clinical and endoscopic efficacy of Mongersen in patients affected by active CD, an interim futility analysis performed during the Phase 3 trial documented lack of efficacy.